The coronavirus pandemic has altered daily life, and it has dominated our conversations. As a result, the general public is now familiar with terms that were previously common only in scientific circles. Phrases like ‘viral load,’ ‘asymptomatic’ and ‘community spread’ have increasingly become parts of everyday discourse as people toss them around matter-of-factly, as if they were topics of conversation well before they were used to describe our current circumstances.
There’s another term — an abbreviation, to be exact — that many have encountered as they seek information on the pandemic and a crucial component of it: PCR, which stands for polymerase chain reaction. The process is commonly used in laboratory settings, but many people now know it as the process behind the gold standard in testing for COVID-19. The term is only recently common parlance, but the process and equipment used for it have been fixtures on college campuses for quite some time.
“PCR machines are known by people at universities and at research labs,” said Liz Rulli, associate vice president for research. “We have some of our student workers say, ‘Oh yeah, I did PCR back in my biology class.’”

With some built-in infrastructure and know-how, an infusion of equipment, partnerships and a massive institution-wide effort, Notre Dame established its own PCR testing lab on campus in the fall. Now, as the University begins the Spring semester, a new certification for the lab signals its readiness to be the primary method of testing for the Notre Dame community.
“Early in the pandemic, it was pretty clear testing was going to a big issue,” said Michael Pfrender, professor of biological sciences and director of Notre Dame’s Genomics and Bioinformatics Core Facility. Pfrender began discussing the feasibility of conducting a test for COVID-19 on campus in the spring of 2020.
“But it wasn’t at all clear at that point that it was something that we needed to do on campus, or that we wanted to do as an institution. Did we want to have something here? It wasn’t until July that it became clear that we really needed to erect a facility on campus that we could really tailor to our student, staff and faculty population,” Pfrender said.

“There was interest in doing a test on our campus in our labs, but we didn’t know what test protocol we could do,” Rulli added. “Yale was coming out with what they called saliva direct, as was the University of Illinois, so there were a lot of things.”
One of the architects of the University of Illinois’ program is Paul Hegenrother, Notre Dame class of ‘94. The Illinois program was heralded as a leading example of efficient mass testing of a student population. In the Fall semester, Illinois tested each of its 50,000 students once a week. Hegenrother was able to share some of the components of the Illinois plan through a non-disclosure agreement, providing key insight and expertise into developing a saliva-based testing protocol at Notre Dame.
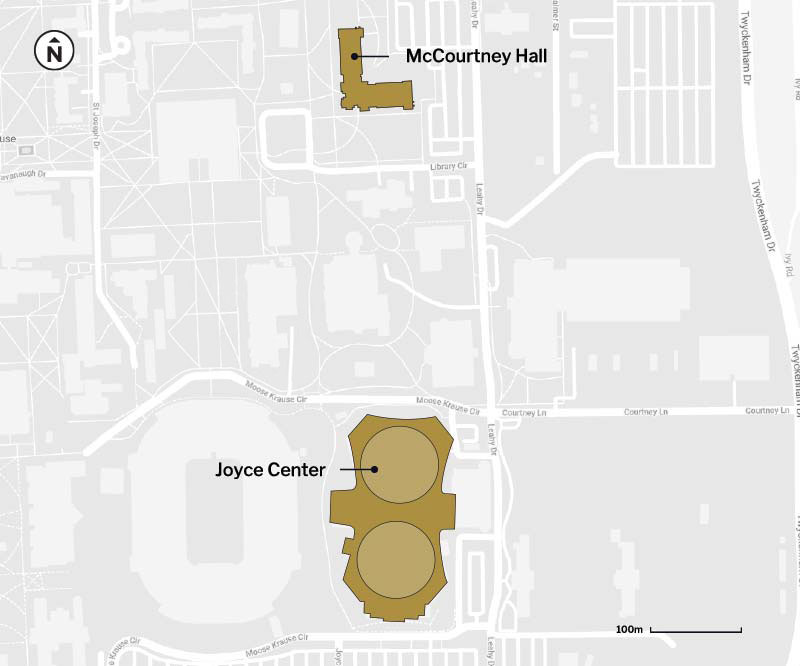
“You need space, you need equipment and you need staff and personnel,” said Pfrender. “We were lucky that Notre Dame Research helped us secure some space in McCourtney Hall that was already well-suited to the task. We had some building modifications done, but we were able to get a lab up and running really rapidly.”



This, despite the challenge of securing enough lab materials to actually conduct the tests. With the entire nation scrambling to set up testing facilities, these items were in short supply. Nevertheless, by September the lab was conducting PCR tests on saliva samples from people within the campus community. Workflows and operating procedures were refined with learned experience and with continued consultation from partners including the University of Illinois.
The test conducted at the lab is a quantitative PCR test. It works by collecting samples from the University Testing Center at the Joyce Center on campus. Participants drool a small amount of saliva into a tube, which is sealed and taken to the lab in McCourtney Hall, some 500 meters away. There, individual samples are first given a heat treatment to inactivate the live virus and placed into a laboratory machine that reads diagnostic genes associated with COVID-19. The samples are not pooled. Each sample is analyzed individually.
Saliva testing is at least as effective as detecting COVID-19 as nasal swabbing, according to a study published by the New England Journal of Medicine. Rulli said the University conducted its own confirmatory follow-up in the Fall semester. (The University’s saliva test has false positive and false negative rates below 1%, on par with the commercial nasal swab test the University also administers which is processed at LabCorp, an off-campus partner.)
The follow-up testing was not just a check on the accuracy of the lab’s test; it was necessary in part because at the time, the lab was not certified to provide a diagnostic result. During the Fall semester, participants in saliva testing received a notice that action in the form of a follow-up nasal test was required (meaning the test had found COVID-19 in their saliva sample), or that no further action was needed (indicating a negative result). Clearance to provide diagnostic results is managed by the federal government’s Clinical Laboratory Improvement Amendments (CLIA) program. The program is a set of regulations meant to provide uniformity in protocols for labs conducting a variety of tests, including those for COVID-19. The University began seeking CLIA certification soon after its lab was established.
“The application part is reasonably straightforward,” said John Lloyd, associate general counsel at Notre Dame. Lloyd guided the University through the application and certification process. “We had to generate a not-too-cumbersome amount of paperwork, but the main thing is thinking about the backend of this so that we have the policies and procedures in place that will pass scrutiny.”
CLIA certification is common in higher education among institutions with medical programs. Those universities have an advantage when it comes to obtaining certification to provide COVID-19 diagnostic results.
“A medical school can do it, a vet school can do it,” Rulli explained, “but we had to do a brand new CLIA. University Health Services has one, but it’s for point-of-care strep tests, which is a non-complex test. Ours [for COVID-19] is a high-complexity test. So we had to get our own CLIA and do all our own paperwork. We couldn’t leverage off of something that we already had from a regulatory perspective like some of the others could do.”
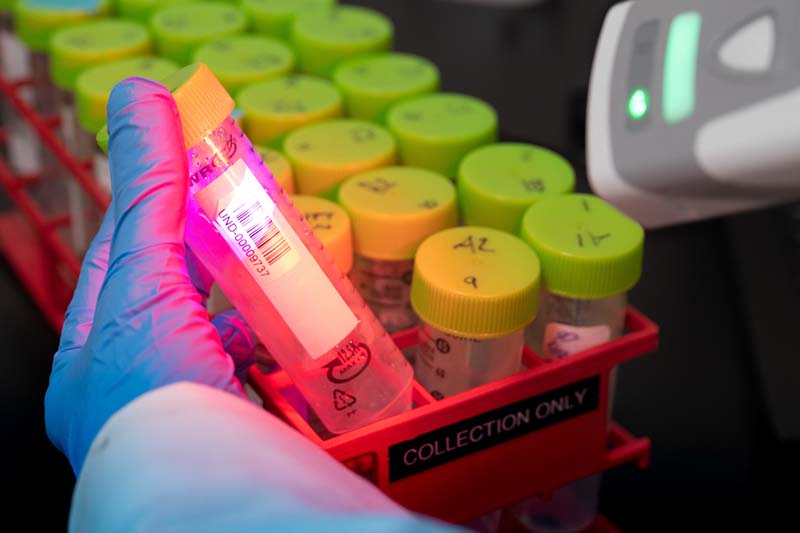
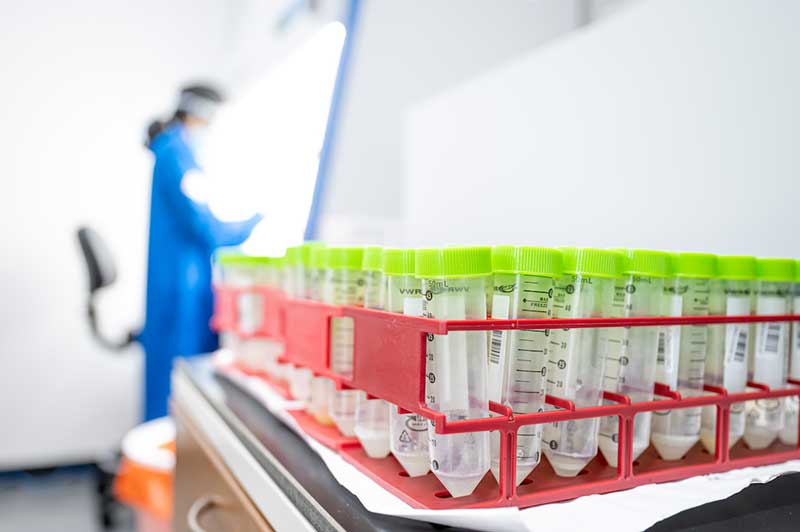
The University obtained its CLIA certification in December, and will be audited by the Indiana State Department of Health (the federal government’s delegated authority) in the coming weeks. The process included a partnership with the South Bend Medical Foundation, which is helping to serve in an advisory role. In the meantime, the lab can provide diagnostic results, even while the methodology and experience for the test participants remains essentially the same as it was in Fall semester.
“They’ll have that diagnostic result now,” Lloyd said. “In some ways it’s not going to be much different from the testing that they were seeing in the fall, except we now have the benefit of having something that they can take and say, ‘Listen, I have the diagnostic test results that are helpful for me for travel plans,’ or for other reasons.”
While the process will remain the same for participants, the volume of testing will change dramatically. The lab started by conducting approximately 200 tests a day, a number gradually increased as part of a surveillance testing strategy that still included a significant number of nasal-swab tests. During the Spring semester, the lab will conduct nearly 11,000 surveillance tests a week as part of an enhanced strategy that requires undergraduate and professional students to be tested once a week, and on-campus faculty, staff and graduate students once every two weeks.

Participants are usually notified of their test results within 24 hours and stats on trends in the campus community are updated each day, a testament to the work of other Notre Dame entities, such as the Office of Information Technology, Center for Research Computing, and Children’s Environmental Health Initiative. The quick turnaround is critical in identifying and stopping outbreaks before they occur, and makes it possible to focus on a certain area of the campus population should the need arise, something the University calls supplemental testing. The ramp-up is possible thanks to an infusion of equipment and a team dedicated solely to making sure the supply chain for testing materials remains intact, which Pfrender said is still one of the biggest potential vulnerabilities of the whole operation, as the nation continues to grapple with the pandemic.
Other challenges related to the pandemic exist, such as the presence of colder weather leading to more indoor activity. Yet amid these challenges are reasons for optimism: continued vaccinations, an increased understanding of the virus, and indeed the capacity for robust testing of the campus population with a quick diagnostic result thanks to CLIA certification. The latter will be a testament long after COVID-19 is a leading topic of conversation.
“Back in March, when we first started asking about testing, it was driven by a desire from people in my group who asked, ‘Can’t we do something?’” Pfrender said. “It’s been really satisfying to think we contributed to keeping everybody safe and keeping the University open.
“But the thing I’m most struck by is how this incredibly complex testing machine has come together. Every day I’m impressed by the level of dedication and the capabilities of people I work with. To see this concerted effort has been an amazing experience for me and given me a much better appreciation for the capabilities of the University to meet new challenges.”



